ATP synthase is a huge molecular complex (>500,000 daltons) embedded in the inner membrane of mitochondria. Its function is to convert the energy of protons (H+) moving down their concentration gradient into the synthesis of ATP. Protons moving through this machine (also known as Complex V) convert a molecule of ADP and Pi (inorganic phosphate) into a molecule of ATP. One ATP synthase complex can generate >100 molecules of ATP each second.
ATP synthase can be separated into 2 parts:
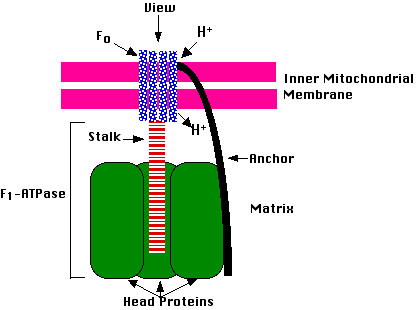
When the F1-ATPase is isolated in vitro, it catalyzes the hydrolysis of ATP to ADP and Pi (which is why it is called the F1-ATPase). While it is doing so, the central portion of Fo attached to the stalk rotates rapidly in a counter-clockwise direction (as viewed from above).
In the intact mitochondrion, the protons that have accumulated in the intermembrane space enter the Fo complex and exit from it into the matrix. The energy they give up as they travel down their concentration gradient rotates Fo and its stalk (at ~6000 rpm) in a clockwise direction. As it does so, it induces repeating conformational changes in the head proteins that enable them to convert ADP and Pi into ATP. (In the figure, two of the three dimers that make up the head proteins have been pulled aside to reveal the stalk inserted in their center.)
In both these cases, the machine is converting chemical energy
But this remarkable device can be made to do the reverse, converting mechanical energy (turning of the motor) into chemical energy.
A group of Japanese scientists interested in nano-machines have succeeded in attaching magnetic beads to the stalks of the F1-ATPase isolated in vitro.
Then using a rotating magnetic field they were able to make the stalks rotate. When rotated in a clockwise direction, the F1-ATPase synthesized ATP from ADP and Pi in the surrounding medium — at a rate of about 5 molecules per second! (When rotating the stalks in the counter-clockwise direction, or not rotating them at all, ATP was hydrolyzed into ADP and Pi.)
Their achievement was reported in Itoh, H., et al., Nature, 29 January 2004.
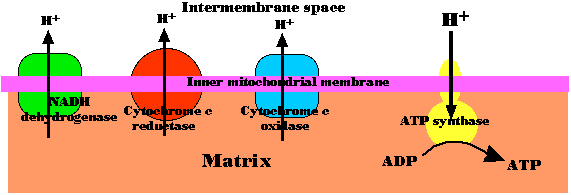
Normally, for each rotation of the stalk, 8 protons (H+) pass into the matrix and 3 molecules of ATP are produced.
But there are rare cases where a mutation in a gene encoding one of the proteins that make up ATP synthase reduces its efficiency.
A recent issue of The New England Journal of Medicine (Ganetzky, R. D. et al, NEJM 387:1395, 13 October, 2022) describes two identical twin boys with unexplained appetites much greater than normal for their age and resulting in an intake of calories 3 times greater than would normally be needed.
Despite the large intake of calories, they failed to gain the weight normal for their age.
Their breathing rates were elevated as was the oxygen consumption by their cells when tested in culture.
They both had periodic fever with excessive sweating but with no sign of infection.
Gene sequencing revealed that each boy had the same point mutation in the gene (ATP5F1B) encoding one of the head proteins in their ATP synthase.
The alteration in the protein structure lessened the efficiency of coupling proton flow to ATP synthesis resulting in the unused energy being converted to heat.| Link to another example of proton flow into the matrix being converted into heat instead of ATP. [Link] |
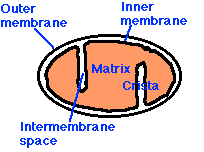
| Return to discussion of cellular respiration. |
| Welcome&Next Search |