The electronegativity of an atom is a measure of its affinity for electrons.
The atoms of the various elements differ in their affinity for electrons.
This image distorts the conventional periodic table of the elements so that the greater the electronegativity of an atom, the higher its position in the table.
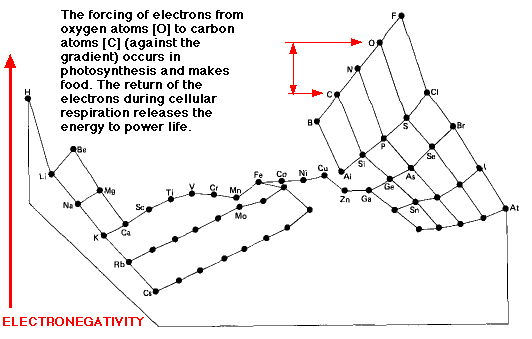
Although fluorine (F) is the most electronegative element, it is the electronegativity of runner-up oxygen (O) that is exploited by life.
- The shuttling of electrons between carbon (C) and oxygen (O) atoms powers life.
- Moving electrons against the gradient (O to C) — as occurs in photosynthesis — requires energy (and stores it).
- Moving electrons down the gradient (C to O) — as occurs in cellular respiration — releases energy.
- The relative electronegativity of two interacting atoms also plays a major part in determining what kind of chemical bond forms between them.
- There is a large difference in electronegativity, so
- the chlorine atom takes an electron from the sodium atom
- converting the atoms into ions (Na+) and (Cl−).
- These are held together by their opposite electrical charge forming ionic bonds.
- Each sodium ion is held by 6 chloride ions while each chloride ion is, in turn, held by 6 sodium ions.
- Result: a crystal lattice (not molecules) of common table salt (NaCl).

Example 2: Carbon (C) and Hydrogen (H) = Covalent Bond
- There is only a small difference in electronegativity, so
- the two atoms share the electrons.
- Result: a covalent bond (depicted as C:H or C-H).
- The atoms are held together by their mutual affinity for their shared electrons.
- An array of atoms held together by covalent bonds forms a true molecule.
Example 3: Hydrogen (H) and Oxygen (O) = Polar Covalent Bond
- Here there is a moderate difference in electronegativity, causing
- the oxygen atom to pull the electron of the hydrogen atom closer to itself.
- Result: a polar covalent bond.
- Oxygen does this with 2 hydrogen atoms to form a molecule of water.
Molecules, like water, with polar covalent bonds
29 January 2011

