Perhaps the first experiment designed to explore the nature of photosynthesis was that reported by the Dutch physician van Helmont in 1648. Some years earlier, van Helmont had placed in a large pot exactly 200 pounds (91 kg) of soil that had been thoroughly dried in an oven. Then he moistened the soil with rain water and planted a 5-pound (2.3 kg) willow shoot in it. He then placed the pot in the ground and covered its rim with a perforated iron plate. The perforations allowed water and air to reach the soil but lessened the chance that dirt or other debris would be blown into the pot from the outside.
For five years, van Helmont kept his plant watered with rain water or distilled water. At the end of that time, he carefully removed the young tree and found that it had gained 164 pound, 3 ounces (74.5 kg). (This figure did not include the weight of the leaves that had been shed during the previous four autumns.) He then redried the soil and found that it weighed only 2 ounces (57 g) less that the original 200 pounds (91 kg). Faced with these experimental facts, van Helmont theorized that the increase in weight of the willow arose from the water alone. He did not consider the possibility that gases in the air might be involved.
The first evidence that gases participate in photosynthesis was reported by Joseph Priestley in 1772. He knew that if a burning candle is placed in a sealed chamber, the candle soon goes out. If a mouse is then placed in the chamber, it soon suffocates because the process of combustion has used up all the oxygen in the air — the gas on which animal respiration depends. However, Priestley discovered that if a plant is placed in an atmosphere lacking oxygen, it soon replenishes the oxygen, and a mouse can survive in the resulting mixture. Priestley thought (erroneously) that it was simply the growth of the plant that accounted for this.
It was another Dutch physician, Ingen-Housz, who discovered in 1778 that the effect observed by Priestley occurred only when the plant was illuminated. A plant kept in the dark in a sealed chamber consumes oxygen just as a mouse (or candle) does.
Ingen-Housz also demonstrated that only green parts of plants liberated oxygen during photosynthesis. Nongreen plant structure, such as woody stems, roots, flowers, and fruits actually consume oxygen in the process of respiration. We now know that this is because photosynthesis can go on only in the presence of the green pigment chlorophyll.
CO2 + H2O → (CH2O) + O2
(The parentheses around the CH2O signify that no specific molecule is being indicated but, instead, the ratio of atoms in some carbohydrate, e.g., glucose, C6H12O6.) The equation also indicates that the ratio of carbon dioxide consumed to oxygen release is 1:1, a finding that was carefully demonstrated in the years following Senebier's work. Using glucose as the carbohydrate product, we can write the equation for photosynthesis as
6CO2 + 6H2O → C6H12O6 + 6O2
 The above equation shows the relationship between the substances used in and produced by the process. It tells us nothing about the intermediate steps. That photosynthesis does involve at least two quite distinct processes became apparent from the experiments of the British plant physiologist F. F. Blackman. His results can easily be duplicated by using the setup on the left. The green water plant Elodea (available wherever aquarium supplies are sold) is the test organism. When a sprig is placed upside down in a dilute solution of NaHCO3 (which serves as a source of CO2) and illuminated with a flood lamp, oxygen bubbles are soon given off from the cut portion of the stem. One then counts the number of bubbles given off in a fixed interval of time at each of several light intensities. Plotting these data produces a graph like the one below.
The above equation shows the relationship between the substances used in and produced by the process. It tells us nothing about the intermediate steps. That photosynthesis does involve at least two quite distinct processes became apparent from the experiments of the British plant physiologist F. F. Blackman. His results can easily be duplicated by using the setup on the left. The green water plant Elodea (available wherever aquarium supplies are sold) is the test organism. When a sprig is placed upside down in a dilute solution of NaHCO3 (which serves as a source of CO2) and illuminated with a flood lamp, oxygen bubbles are soon given off from the cut portion of the stem. One then counts the number of bubbles given off in a fixed interval of time at each of several light intensities. Plotting these data produces a graph like the one below.
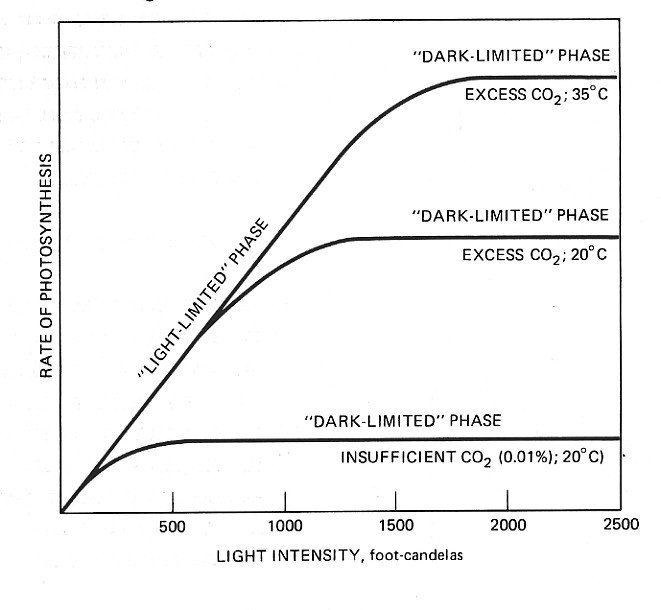
Since the rate of photosynthesis does not continue to increase indefinitely with increased illumination, Blackman concluded that at least two distinct processes are involved: one, a reaction that requires light and the other, a reaction that does not. This latter is called a "dark" reaction although it can go on in the light. Blackman theorized that at moderate light intensities, the "light" reaction limits or "paces" the entire process. In other words, at these intensities the dark reaction is capable of handling all the intermediate substances produced by the light reaction. With increasing light intensities, however, a point is eventually reached when the dark reaction is working at maximum capacity. Any further illumination is ineffective, and the process reaches a steady rate.
This interpretation is strengthened by repeating the experiment as a somewhat higher temperature. Most chemical reactions proceed more rapidly at higher temperatures (up to a point). At 35°C, the rate of photosynthesis does not level off until greater light intensities are present. This suggest that the dark reaction is now working faster. The fact that at low light intensities the rate of photosynthesis is no greater at 35°C than at 20°C also supports the idea that it is a light reaction that is limiting the process in this range. Light reactions depend, not on temperature, but simply on the intensity of illumination.
The increased rate of photosynthesis with increased temperature does not occur if the supply of CO2 is limited. As the figure shows, the overall rate of photosynthesis reaches a steady value at lower light intensities if the amount of CO2 available is limited. Thus CO2 concentration must be added as a third factor regulating the rate at which photosynthesis occurs. As a practical matter, however, the concentration available to terrestrial plants is simply that found in the atmosphere: 0.035%.It was the American microbiologist Van Niel who first glimpsed the role that light plays in photosynthesis. He studied photosynthesis in purple sulfur bacteria. These microorganisms synthesize glucose from CO2 as do green plants, and they need light to do so. Water, however, is not the starting material. Instead they use hydrogen sulfide (H2S). Furthermore, no oxygen is liberated during this photosynthesis but rather elemental sulfur. Van Niel reasoned that the action of light caused a decomposition of H2S into hydrogen and sulfur atoms. Then, in a series of dark reactions, the hydrogen atoms were used to reduce CO2 to carbohydrate:
CO2 + 2H2S → (CH2O) + H2O + 2S
Van Niel envisioned a parallel to the process of photosynthesis as it occurs in green plants. There the energy of light causes water to break up into hydrogen and oxygen. The hydrogen atoms are then used to reduce CO2 in a series of dark reactions:
CO2 + 2H2O → (CH2O) + H2O + O2
If this theory is correct, then it follows that all of the oxygen released during photosynthesis comes from water just as all the sulfur produced by the purple sulfur bacteria comes from H2S. This conclusion directly contradicts Senebier's theory that the oxygen liberated in photosynthesis comes from the carbon dioxide. If Van Niel's theory is correct, then the equation for photosynthesis would have to be rewritten:
6CO2 + 12H2O → C6H12O6 + 6 H2O + 6O2
In science, a theory should be testable. By deduction, one can make a prediction of how a particular experiment will come out if the theory is sound. In this case, the crucial experiments needed to test the two theories had to await the time when the growth of atomic research made it possible to produce isotopes other than those found naturally or in greater concentrations than are found naturally.
In air, water and other natural materials containing oxygen, 99.76% of the oxygen atoms are 16O and only 0.20% of them are the heavier isotope 18O. In 1941, Samuel Ruben and his coworkers at the University of California were able to prepare specially "labeled" water in which the 0.85% of the molecules contained 18O atoms. When this water was supplied to a suspension of photosynthesizing algae, the proportion of 18O in the oxygen gas that was evolved was 0.85%, the same as that of the water supplied, and not simply the 0.20% found in all natural samples of oxygen (and its compounds like CO2).
| % 18O FOUND IN | ||||
| EXPERIMENT | H2O | CO2 | O2 | |
| 1. | START | 0.85 | 0.20 | — |
| FINISH | 0.85 | 0.61* | 0.86 | |
| 2. | START | 0.20 | 0.68 | — |
| FINISH | 0.20 | 0.57 | 0.20 | |
These results clearly demonstrated that Senebier's interpretation was in error. If all the oxygen liberated during photosynthesis comes from the carbon dioxide, we would expect the oxygen evolved in Ruben's experiment to contain simply the 0.20% found naturally. If, on the other hand, both the carbon dioxide and the water contribute to the oxygen released, we would expect its isotopic composition to have been some intermediate figure. In fact, the isotopic composition of the evolved oxygen was the same as that of the water used.
Ruben and his colleagues also prepared a source of carbon dioxide that was enriched in 18O atoms. When algae carried out photosynthesis using this material and natural water, the oxygen that was given off was not enriched in 18O. It contained simply the 0.20% 18O found in the natural water used. The heavy atoms presumably became incorporated in the other two products (carbohydrate and by-product water).
These experiments lent great support to Van Niel's idea that one function of light in photosynthesis was the separation of the hydrogen and oxygen atoms of water molecules. But there remained to work out just how the hydrogen atoms were made available to the dark reactions. The process is described in Photosynthesis: The Role of Light.
The details of the dark reactions of photosynthesis are described in Photosynthesis: Pathway of Carbon Fixation
| Welcome&Next Search |