A transgenic animal is one that carries a foreign gene that has been deliberately inserted into its genome. The foreign gene is constructed using recombinant DNA methodology. In addition to the gene itself, the DNA usually includes other sequences to enable it
- to be incorporated into the DNA of the host and
- to be expressed correctly by the cells of the host.
- Transgenic sheep and goats have been produced that express foreign proteins in their milk.
- Transgenic chickens are now able to synthesize human proteins in the "white" of their eggs.
These animals should eventually prove to be valuable sources of proteins for human therapy.
| In July 2000, researchers from the team that produced Dolly reported success in producing transgenic lambs in which the transgene had been inserted at a specific site in the genome and functioned well. [More] |
Transgenic mice have provided the tools for exploring many biological questions.
Normal mice cannot be infected with polio virus. They lack the cell-surface molecule that, in humans, serves as the receptor for the virus. So normal mice cannot serve as an inexpensive, easily-manipulated model for studying the disease.
However, transgenic mice expressing the human gene for the polio virus receptor
- can be infected by polio virus and even
- develop paralysis and other pathological changes characteristic of the disease in humans.
- transforming embryonic stem cells (ES cells) growing in culture with the desired DNA;
- injecting the desired gene into the pronucleus of a fertilized mouse egg.
Embryonic stem cells (ES cells) are harvested from the inner cell mass (ICM) of mouse blastocysts. They can be grown in culture and retain their full potential to produce all the cells of the mature animal, including its gametes.
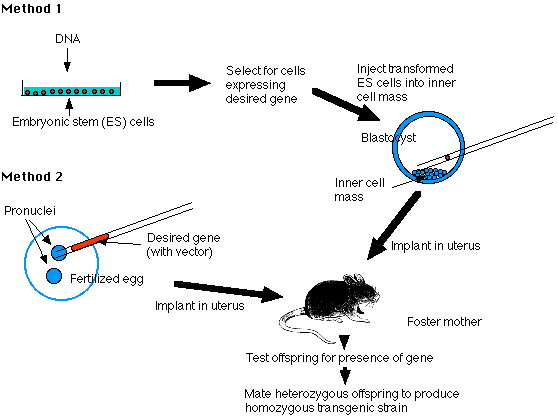 Using recombinant DNA methods, build molecules of DNA containing
Using recombinant DNA methods, build molecules of DNA containing
- the gene you desire (e.g., the insulin gene);
- vector DNA to enable the molecules to be inserted into host DNA molecules;
- promoter and enhancer sequences to enable the gene to be expressed by host cells.
Expose the cultured cells to the DNA so that some will incorporate it.
3. Select for successfully transformed cells. [Method]
- Prepare a pseudopregnant mouse (by mating a female mouse with a vasectomized male). The stimulus of mating elicits the hormonal changes needed to make her uterus receptive.
- Transfer the embryos into her uterus.
- Hope that they implant successfully and develop into healthy pups (no more than one-third will).
- Remove a small piece of tissue from the tail and examine its DNA for the desired gene. No more than 10–20% will have it, and they will be heterozygous for the gene.
- Mate two heterozygous mice and screen their offspring for the 1 in 4 that will be homozygous for the transgene.
- Mating these will found the transgenic strain.
The Pronucleus Method (Method "2")
- Harvest freshly fertilized eggs before the sperm head has become a pronucleus.
- Inject the male pronucleus with your DNA.
- When the pronuclei have fused to form the diploid zygote nucleus, allow the zygote to divide by mitosis to form a 2-cell embryo.
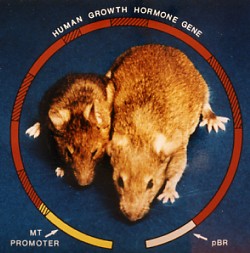 This image (courtesy of R. L. Brinster and R. E. Hammer) shows a transgenic mouse (right) with a normal littermate (left). The giant mouse developed from a fertilized egg transformed with a recombinant DNA molecule containing:
The levels of growth hormone in the serum of some of the transgenic mice were several hundred times higher than in control mice.
The early vectors used for gene insertion could, and did, place the gene (from one to 200 copies of it) anywhere in the genome. However, if you know some of the DNA sequence flanking a particular gene, it is possible to design vectors that replace that gene. The replacement gene can be one that
This image (courtesy of R. L. Brinster and R. E. Hammer) shows a transgenic mouse (right) with a normal littermate (left). The giant mouse developed from a fertilized egg transformed with a recombinant DNA molecule containing:
The levels of growth hormone in the serum of some of the transgenic mice were several hundred times higher than in control mice.
The early vectors used for gene insertion could, and did, place the gene (from one to 200 copies of it) anywhere in the genome. However, if you know some of the DNA sequence flanking a particular gene, it is possible to design vectors that replace that gene. The replacement gene can be one that
- restores function in a mutant animal or
- knocks out the function of a particular locus.
In either case, targeted gene insertion requires
- the desired gene
- neor, a gene that encodes an enzyme that inactivates the antibiotic neomycin and its relatives, like the drug G418, which is lethal to mammalian cells;
- tk, a gene that encodes thymidine kinase, an enzyme that phosphorylates the nucleoside analog ganciclovir. DNA polymerase fails to discriminate against the resulting nucleotide and inserts this nonfunctional nucleotide into freshly-replicating DNA. So ganciclovir kills cells that contain the tk gene.
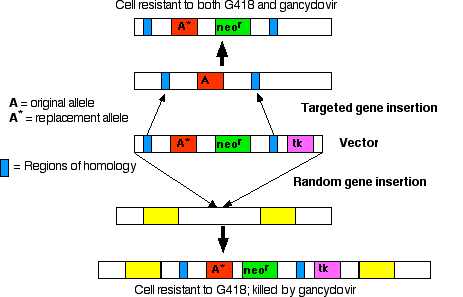 Treat culture of ES cells with preparation of vector DNA.
Treat culture of ES cells with preparation of vector DNA.
Results:
- Most cells fail to take up the vector; these cells will be killed if exposed to G418.
- In a few cells: the vector is inserted randomly in the genome. In random insertion, the entire vector, including the tk gene, is inserted into host DNA. These cells are resistant to G418 but killed by gancyclovir.
- In still fewer cells: homologous recombination occurs. Stretches of DNA sequence in the vector find the homologous sequences in the host genome, and the region between these homologous sequences replaces the equivalent region in the host DNA.
Culture the mixture of cells in medium containing both G418 and ganciclovir.
- The cells (the majority) that failed to take up the vector are killed by G418.
- The cells in which the vector was inserted randomly are killed by gancyclovir (because they contain the tk gene).
- This leaves a population of cells transformed by homologous recombination (enriched several thousand fold).
Inject these into the inner cell mass of mouse blastocysts.
Knockout Mice: What do they teach us?
If the replacement gene (A* in the diagram) is nonfunctional (a "null" allele), mating of the heterozygous transgenic mice will produce a strain of "knockout mice" homozygous for the nonfunctional gene (both copies of the gene at that locus have been "knocked out").
Knockout mice are valuable tools for discovering the function(s) of genes for which mutant strains were not previously available. Two generalizations have emerged from examining knockout mice:
- Knockout mice are often surprisingly unaffected by their deficiency. Many genes turn out not to be indispensable. The mouse genome appears to have sufficient redundancy to compensate for a single missing pair of alleles.
- Most genes are pleiotropic. They are expressed in different tissues in different ways and at different times in development.
While "housekeeping" genes are expressed in all types of cells at all stages of development, other genes are normally expressed in only certain types of cells when turned on by the appropriate signals (e.g. the arrival of a hormone).
To study such genes, one might expect that the methods described above would work. However, it turns out that genes that are only expressed in certain adult tissues may nonetheless be vital during embryonic development. In such cases, the animals do not survive long enough for their knockout gene to be studied.
Fortunately, there are now techniques with which transgenic mice can be made where a particular gene gets knocked out in only one type of cell.
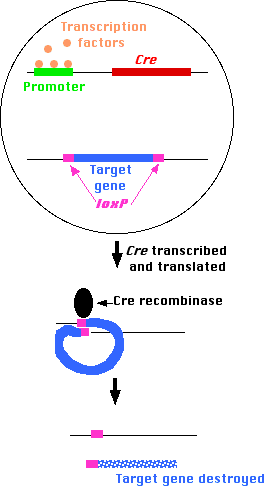
One of the bacteriophages that infects E. coli, called P1, produces an enzyme — designated Cre — that cuts its DNA into lengths suitable for packaging into fresh virus particles. Cre cuts the viral DNA wherever it encounters a pair of sequences designated loxP. All the DNA between the two loxP sites is removed, and the remaining DNA ligated together again (so the enzyme is a recombinase).
Using "Method 1" (above), mice can be made transgenic for
- the gene encoding Cre attached to a promoter that will be activated only when it is bound by the same transcription factors that turn on the other genes required for the unique function(s) of that type of cell;
- a "target" gene, the one whose function is to be studied, flanked by loxP sequences.
In the adult animal,
- those cells that
- receive signals (e.g., the arrival of a hormone or cytokine)
- to turn on production of the transcription factors needed
- to activate the promoters of the genes whose products are needed by that particular kind of cell
will also turn on transcription of the Cre gene. Its protein will then remove the "target" gene under study.
- All other cells will lack the transcription factors needed to bind to the Cre promoter (and/or any enhancers) so the target gene remains intact.
The result: a mouse with a particular gene knocked out in only certain cells.
The Cre/loxP system can also be used to
- remove DNA sequences that block gene transcription. The "target" gene can then be turned on in certain cells or at certain times as the experimenter wishes.
- replace one of the mouse's own genes with a new gene that the investigator wishes to study.
Such transgenic mice are called "knock-in" mice.
Until recently, the transgenes introduced into sheep inserted randomly in the genome and often worked poorly. However, in July 2000, success at inserting a transgene into a specific gene locus was reported. The gene was the human gene for alpha1-antitrypsin, and two of the animals expressed large quantities of the human protein in their milk.
This is how it was done.
Sheep fibroblasts (connective tissue cells) growing in culture were treated with a vector that contained these segments of DNA:
- 2 regions homologous to the sheep COL1A1 gene. This gene encodes Type 1 collagen. (Its absence in humans causes the inherited disease osteogenesis imperfecta.)
This locus was chosen because fibroblasts secrete large amounts of collagen and thus one would expect the gene to be easily accessible in the chromatin.
- A neomycin-resistance gene to aid in isolating those cells that successfully incorporated the vector. [Link to technique]
- The human gene encoding alpha1-antitrypsin.
Some people inherit two non- or poorly-functioning genes for this protein. Its resulting low level or absence produces the disease Alpha1-Antitrypsin Deficiency (A1AD or Alpha1). The main symptoms are damage to the lungs (and sometimes to the liver).
- Promoter sites from the beta-lactoglobulin gene. These promote hormone-driven gene expression in milk-producing cells.
- Binding sites for ribosomes for efficient translation of the beta-lactoglobulin mRNAs.
Successfully-transformed cells were then
- fused with enucleated sheep eggs [Link to description of the method] and
- implanted in the uterus of a ewe (female sheep).
- Several embryos survived until their birth, and two young lambs lived over a year.
- When treated with hormones, these two lambs secreted milk containing large amounts of alpha1-antitrypsin (650 µg/ml; 50 times higher than previous results using random insertion of the transgene).
On June 18, 2003, the company doing this work abandoned it because of the great expense of building a facility for purifying the protein from sheep's milk. Purification is important because even when 99.9% pure, human patients can develop antibodies against the tiny amounts of sheep proteins that remain.
However, another company, GTC Biotherapeutics, has persevered and in June of 2006 won preliminary approval to market a human protein, antithrombin, in Europe. Their protein — the first made in a transgenic animal to receive regulatory approval for human therapy — was secreted in the milk of transgenic goats.
Chickens
- grow faster than sheep and goats and large numbers can be grown in close quarters;
- synthesize several grams of protein in the "white" of their eggs.
Two methods have succeeded in producing chickens carrying and expressing foreign genes.
- Infecting embryos with a viral vector carrying
- the human gene for a therapeutic protein
- promoter sequences that will respond to the signals for making proteins (e.g. lysozyme) in egg white.
- Transforming rooster sperm with a human gene and the appropriate promoters and checking for any transgenic offspring.
Preliminary results from both methods indicate that it may be possible for chickens to produce as much as 0.1 g of human protein in each egg that they lay.
Not only should this cost less than producing therapeutic proteins in culture vessels, but chickens will probably add the correct sugars to glycosylated proteins — something that E. coli cannot do.
Transgenic pigs have also been produced by fertilizing normal eggs with sperm cells that have incorporated foreign DNA. This procedure, called sperm-mediated gene transfer (SMGT) may someday be able to produce transgenic pigs that can serve as a source of transplanted organs for humans. [More]
In the 28 May 2009 issue of Nature, Japanese scientists reported success in creating transgenic marmosets. Marmosets are primates and thus our closest relatives (so far) to be genetically engineered. In some cases, the transgene (for green fluorescent protein) was incorporated into the germline and passed on to the animal's offspring. The hope is that these transgenic animals will provide the best model yet for studying human disease and possible therapies.
23 October 2022

 This image (courtesy of R. L. Brinster and R. E. Hammer) shows a transgenic mouse (right) with a normal littermate (left). The giant mouse developed from a fertilized egg transformed with a recombinant DNA molecule containing:
This image (courtesy of R. L. Brinster and R. E. Hammer) shows a transgenic mouse (right) with a normal littermate (left). The giant mouse developed from a fertilized egg transformed with a recombinant DNA molecule containing:

