Stem Cells
Stem cells are cells that divide by mitosis to form either
- two stem cells, thus increasing the size of the stem cell "pool",
- or
- one daughter that goes on to differentiate, and
- one daughter that retains its stem-cell properties.
How the choice is made is still unknown. However, several genes have been found whose activity prevents a daughter cell from differentiating.
Several adjectives are used to describe the developmental potential of stem cells; that is, the number of different kinds of differentiated cell that they can become.
- Totipotent cells. In mammals, totipotent cells have the potential to become
The only totipotent cells are the fertilized egg and the first 4 or so cells produced by its cleavage (as shown by the ability of mammals to produce identical twins, triplets, etc.).
In mammals, the expression totipotent stem cells is a misnomer — totipotent cells cannot make more of themselves.
- Pluripotent stem cells. These are true stem cells, with the potential to make any differentiated cell in the body (but probably not those of the placenta which is derived from the trophoblast).
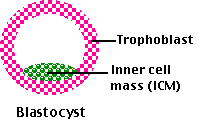
Three types of pluripotent stem cells occur naturally:
- Embryonic Stem Cells (ESCs). These can be isolated from the inner cell mass (ICM) of the blastocyst — the stage of embryonic development when implantation occurs. For humans, excess embryos produced during in vitro fertilization (IVF) procedures are used. Harvesting ESCs from human blastocysts is controversial because it destroys the embryo, which could have been implanted to produce another baby (but often was simply going to be discarded).
- Embryonic Germ Cells. These can be isolated from the precursor to the gonads in aborted fetuses.
- Embryonic Carcinoma Cells. These can be isolated from teratocarcinomas, a tumor that occasionally occurs in a gonad of a fetus. Unlike the other two, they are usually aneuploid.
All three of these types of pluripotent stem cells
- can only be isolated from embryonic or fetal tissue;
- can be grown in culture, but only with special methods to prevent them from differentiating.
In mice and rats, embryonic stem cells (ESCs) can also:
Induced pluripotent stem cells (iPSCs)
Using genetic manipulation in the laboratory, pluripotent stem cells can now be generated from differentiated cells. These induced pluripotent stem cells (iPSCs) are described below.
- Multipotent stem cells. These are true stem cells but can only differentiate into a limited number of types. For example, the bone marrow contains multipotent stem cells that give rise to all the cells of the blood but not to other types of cells. [Discussion]
Multipotent stem cells are found in adult animals; perhaps most organs in the body (e.g., brain, liver, lungs) contain them where they can replace dead or damaged cells. These adult stem cells may also be the cells that — when one accumulates sufficient mutations — produce a clone of cancer cells.
The Dream
Many medical problems arise from damage to differentiated cells.
Examples:
The great developmental potential of stem cells has created intense research into enlisting them to aid in replacing the lost cells of such disorders.
While progress has been slow, some procedures already show promise.
Using multipotent "adult" stem cells.
- culturing human epithelial stem cells and using their differentiated progeny to replace a damaged cornea. This works best when the stem cells are from the patient (e.g. from the other eye). Corneal cells from another person (an allograft) are always at risk of rejection by the recipient's immune system.
- the successful repair of a damaged left bronchus using a section of a donated trachea that was first cleansed of all donor cells and then seeded with the recipient's epithelial cells and cartilage-forming cells grown from stem cells in her bone marrow. So far the patient is doing well and needs no drugs to suppress her immune system.
Using differentiated cells derived from embryonic stem cells (ESCs). Clinical trials are underway to assess the safety of
- injecting retinal cells derived from ESCs
- into the eyes of young people with an inherited form of juvenile blindness;
- into the eyes of adults with age-related macular degeneration.
- inserting capsules containing insulin-secreting cells derived from ESCs or iPSCs into patients with Type 1 diabetes.
One major problem that must be solved before human stem cell therapy becomes a reality is the threat of rejection of the transplanted cells by the host's immune system (if the stem cells are allografts; that is, come from a genetically-different individual).
One way to avoid the problem of rejection is to use stem cells that are genetically identical to the host.
This is already possible in the rare situations when the patient has healthy stem cells in an undamaged part of the body (like the stem cells being used to replace damaged corneas or those used to treat junctional epidermolysis bullosa).
But even where no "autologous" stems cells are available, there may be a solution: using somatic-cell nuclear transfer .
In this technique,
- An egg has its own nucleus removed and replaced by
- a nucleus taken from a somatic (e.g., skin) cell of the donor.
- The now-diploid egg is allowed to develop in culture to the blastocyst stage when
- embryonic stem cells can be harvested and grown up in culture.
- When they have acquired the desired properties, they can be implanted in the donor with no fear of rejection.
Using this procedure it possible to not only grow blastocysts but even have these go on to develop into adult animals — cloning — with a nuclear genome identical to that of the donor of the nucleus. The first successful cloning by SCNT was with amphibians [View procedure]. Later, mammals such as sheep (Dolly), cows, mice and others were successfully cloned.
And in the 11 November 2007 issue of Science, researchers in Oregon reported success with steps 1–4 in rhesus monkeys (primates like us).
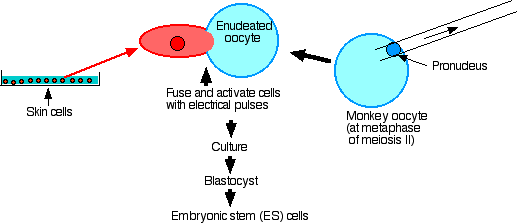
Their procedure:
- Remove the spindle and thus all nuclear material from secondary oocytes at metaphase of meiosis II.
- Fuse each enucleated egg with a skin cell taken from a male monkey.
- Culture until the blastocyst stage is reached.
- Extract embryonic stem cells from the inner cell mass.
- Establish that they have the nuclear genome of the male (but mostly the mitochondrial genome of the female).
- Culture with factors to encourage differentiation: they grew cardiac muscle cells (which contracted), and even neuron-like cells.
- Inject into SCID mice and examine the tumors that formed. These contained cells of all three germ layers: ectoderm, mesoderm, and endoderm.
- However, even after more than 100 attempts, they have not been able to implant their monkey blastocysts in the uterus of a surrogate mother to produce a cloned monkey.
This should reassure people who view with alarm the report in May 2013 by the same workers that they have finally succeeded in producing embryonic stem cells (ESCs) using SCNT from differentiated human tissue. The workers assure us that they will not attempt to implant these blastocysts in a surrogate mother to produce a cloned human. And their failure with monkeys suggests that they would fail even if they did try.
While cloning humans still seems impossible, patient-specific ESCs
- could be used in cell-replacement therapy or, failing that,
- provide the material for laboratory study of the basis of — and perhaps treatment of — genetic diseases.
Whether they will be more efficient and more useful than induced pluripotent stem cells [below] remains to be seen.
- Imprinted Genes.
Sperm and eggs each contain certain genes that carry an "imprint" identifying them later in the fertilized egg as being derived from the father or mother respectively.
Creating an egg with a nucleus taken from an adult cell may not allow a proper pattern of imprinting to be established.
When the diploid adult nucleus is inserted into the enucleated egg (at least those of sheep and mice), the new nucleus becomes "reprogrammed". What reprogramming actually means still must be learned, but perhaps it involves the proper methylation and demethylation of imprinted genes. For example, the inactive X chromosome in adult female cells must be reactivated in the egg, and this actually seems to happen.
- Aneuploidy.
In primates (in contrast to sheep, cattle, and mice), the process of removing the resident nucleus causes molecules associated with the centrosome to be lost as well. Although injecting a donor nucleus allows mitosis to begin, spindle formation may be disrupted, and the resulting cells fail to get the correct complement of chromosomes (aneuploidy).
- Somatic Mutations.
This procedure also raises the spectre of amplifying the effect(s) of somatic mutations. [Link to discussion]
In other words, mutations that might be well-tolerated in a single somatic cell of the adult (used to provide the nucleus) might well turn out to be quite harmful when they become replicated in a clone of cells injected later into the patient.
Furthermore even if the initial stem cells are free of deleterious mutations, as they proliferate in culture, they can acquire mutations. Recently (2017) it has been shown that human embryonic stem cells grown in culture gradually acquire mutations in the TP53 gene — the most frequent "driver" gene in human cancers. The acquisition of a mutant TP53 seems to provide a growth advantage in the culture so that as time goes on, this subclone comes to dominate the population.
This phenomenon should provide a cautionary note in proposals to inject humans with cultures stem cells.
- Political Controversy.
The goal of this procedure (which is often called therapeutic cloning even though no new individual is produced) is to culture a blastocyst that can serve as a source of ES cells.
But that same blastocyst could theoretically be implanted in a human uterus and develop into a baby that was genetically identical to the donor of the nucleus. In this way, a human would be cloned.
And in fact, Dolly and other animals are now routinely cloned this way. Link to a description.
The spectre of this is so abhorrent to many that they would like to see the procedure banned despite its promise for helping humans.
In fact, many are so strongly opposed to using human blastocysts — even when produced by nuclear transfer — that they would like to limit stem cell research to adult stem cells (even though these are only multipotent).
Induced pluripotent stem cells (iPSCs)
A promising alternative to the use of embryonic stem cells in human therapy are recently-developed methods of genetically reprogramming the nuclei of differentiated adult cells so that they regain the pluripotency of embryonic stem cells (ESCs).
In June 2007, three laboratories reported that introducing extra copies of only 4 genes into adult mouse skin cells (fibroblasts) enables them to regain the properties of ESCs. When these cells, named induced pluripotent stem cells (iPSCs for short), were placed in mouse blastocysts, they participated in building all the tissues of the chimeric mice that resulted. (When placed in tetraploid (4n) blastocysts — unable by themselves to develop normally — embryos were formed that thus were clones of the skin cell donor.) The four genes: c-Myc, Sox2, Oct3/4, Klf4.
| By 2009, several labs had succeeded in producing fertile adult mice from iPSCs derived from mouse embryonic fibroblasts. This shows that iPSCs are just a capable of driving complete development (pluripotency) as embryonic stem cells. |
Reprogramming works in humans, too! Using the same four genes, the Yamanaka lab in Japan reported on 20 November 2007, that they now had reprogrammed human skin cells to become induced pluripotent stem cells (iPSCs). And the Thomson lab in Wisconsin accomplished the same thing using SOX2, OCT4, NANOG, and LIN28.
|
Further evidence of the remarkable role played by these few genes is the finding that during normal embryonic development of the zebrafish, the same or similar genes (SoxB1, Oct4, Nanog) are responsible for turning on the genes of the zygote. Earlier in development of the blastula, all the genes being expressed (including these) are the mother's — mRNAs and proteins that the mother deposited in the unfertilized egg [More]. It makes sense that the same proteins that can reprogram a differentiated cell into a pluripotent state (iPSCs) are those that produce the pluripotent cells of the early embryo.
|
These achievements open the possibility of
- creating cells for laboratory study of the basis of genetic diseases.
Examples: researchers have succeeded in deriving iPSCs from
- patients with amyotrophic lateral sclerosis (ALS, "Lou Gehrig's disease"), and then causing them to differentiate into motor neurons (the cells affected in the disease) for study of their properties;
- the skin cells of a patient with an inherited heart disease (long QT syndrome) and causing these to differentiate into beating heart cells for study in the laboratory.
- The Jaenisch lab reported in the 6 March 2009 issue of Cell that they have succeeded in making iPSCs (they call them hiPSCs) from fibroblasts taken from patients with Parkinson's disease. The cells were then differentiated into dopamine-releasing cells — the cells lacking in this disease. What is particularly exciting is that they accomplished this after using the Cre-lox system to remove all the genes (e.g., SOX2, OCT4, KLF4) needed for reprogramming the fibroblasts to an embryonic-stem-cell-like condition.
- Since that report, other laboratories — using other methods — have also created iPSCs from which all foreign DNA (vector and transgenes) has been removed. Not only should such cells be safer to use in therapy, but these results show that the stimulus to reprogram a differentiated cell into a pluripotent state need only be transitory.
- creating patient-specific cell transplants — avoiding the threat of immunological rejection — that could be used for human therapy.
Therapy with iPSCs has already been demonstrated in mice. Three examples:
1. The Jaenisch lab in Cambridge, MA reported (in Science, 21 December 2007) that they had successfully treated knock-in mice that make sickle-cell hemoglobin with the human βS genes (and show many of the signs of sickle-cell disease in humans) by
- harvesting some fully-differentiated fibroblasts from a sickle-cell mouse;
- reprogramming these to become iPSCs by infecting them with Oct4, Sox2, Klf4, and c-Myc;
- then removing (using the Cre-lox system) the c-Myc to avoid the danger of this oncogene later causing cancer in the recipient mice;
- replacing the βS genes in the iPSCs with normal human βA genes;
- coaxing, with a cocktail of cytokines, these iPSCs to differentiate in vitro into hematopoietic (blood cell) precursors;
- injecting these into sickle-cell mice that had been irradiated to destroy their own bone marrow (as is done with human bone marrow transplants). (Although the recipient mice were different animals from the fibroblast donor, they were of the same inbred strain and thus genetically the same — like identical human twins. So the procedure fully qualifies as "patient-specific", i.e., with no danger of the injected cells being rejected by the recipient's immune system.)
The result: all the signs of sickle-cell disease (e.g., anemia) in the treated animals showed marked improvement.
2. In the 25 July 2013 issue of Nature, a team of Japanese scientists report that they were able to manufacture three-dimensional buds of human liver cells.
Their process:
- create human iPSCs from human fibroblasts using the techniques described above;
- treat these with the substances needed for them to differentiate in liver cell precursors;
- culture these with a mixture of human endothelial cells and mesenchymal stem cells (to mimic the conditions that occur in normal embryonic development of the liver);
- implant the resulting solid masses (buds) of liver-like cells into immunodeficient mice.
The result: the implanted buds developed a blood supply and the mice began to secrete human albumin, human alpha-1-antitrypsin, and to to detoxify injected chemicals just as human livers do.
3. Workers in the Melton lab at Harvard University reported in the 9 October 2014 issue of Cell that they had succeeded in differentiating large numbers of human beta cells from human iPSCs (as well as from human ESCs). When transplanted into diabetic mice, these cells brought their elevated blood sugar levels back down. (In the case of humans with Type 1 diabetes mellitus, even patient-derived beta cells will still be at risk of the same autoimmune rejection that caused the disease in the first place. Tests are underway to see if this can be avoided by putting the insulin-secreting (beta) cells in a semi-permeable capsule that allows insulin to diffuse out but blocks autoimmune T cells from getting in.)
Let us hope that what works in mice can someday be developed into a safe therapy that will work in humans.
Early trials with several patients with diabetes do show promising results.
The general procedure:
- Harvest differentiated cells (e.g., skin cells) from the patient and place them in culture.
- Treat the cells with substances that reprogram them to a stem cell state (iPSCs). These cells can be grown indefinitely; that is, do not not show replicative senescence,
- Treat these iPSCs with substances that cause the cells to differentiate into insulin-secreting (islets) cells.
- Inject these into the patient. Because they are the patients own (autologous) cells, there should not be any danger of their being rejected by the immune system.
One result:
Twelve months after receiving her cells, a woman with Type 1 diabetes no longer needed injections of insulin.
Despite these advances, iPSCs will have to be used cautiously for human therapy. Several groups have found that human iPSCs contain mutations as well as epigenetic patterns (e.g., methylation of their DNA) that are not found in embryonic stem cells. Some of the mutations are also commonly found in cancer cells.
Other approaches being explored
- Embryonic stem cells (ESCs) can be derived from a single cell removed from an 8-cell morula. The success of preimplantation genetic diagnosis (PGD) in humans shows that removing a single cell from the morula does not destroy it — the remaining cells can develop into a blastocyst, implant, and develop into a healthy baby. Furthermore, the single cell removed for PGD can first be allowed to divide with one daughter used for PGD and the other a potential source of an embryonic stem cell line.
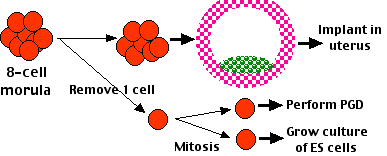
- In altered nuclear transfer (ANT) — a modified version of SCNT (somatic-cell nuclear transfer) — a gene necessary for later implantation (Cdx2 — encoding a homeobox transcription factor) is turned off (by RNA interference) in the donor nucleus before the nucleus is inserted into the egg. The blastocyst that develops
- has a defective trophoblast that cannot implant in a uterus;
- but the cells of the inner cell mass are still capable of developing into cultures of ESCs. (The gene encoding the interfering RNA can then be removed using the Cre/loxP technique.)
- Jose Cibelli and his team at Advanced Cell Technology reported in the 1 February 2002 issue of Science that they had succeeded in
- stimulating monkey oocytes to begin dividing without completing meiosis II (therefore still 2n)
- growing these until the blastocyst stage, from which they were able to harvest
- embryonic stem cells.
If this form of cloning by parthenogenesis works in humans [It does! — success with unfertilized human eggs was reported in June 2007.], it would have
- the advantage that no babies could be produced if the blastocyst should be implanted (two identical genomes cannot produce a viable mammal — probably because of incorrect imprinting);
- the disadvantage that it will only help females (because only they can provide an oocyte!) (But men may have a procedure that works for them — next.)
- On 24 March 2006, Nature published an online report that a group of German scientists had been able to derive pluripotent stem cells from the stem cells that make spermatogonia in the mouse. Both in vitro and when injected into mouse blastocysts, these cells differentiated in a variety of ways including representatives of all three germ layers. If this could work in humans, it would
- provide a source of stem cells whose descendants would be "patient-specific"; that is, could be transplanted back into the donor (men only!) without fear of immune rejection.
- avoid the controversy surrounding the need to destroy human blastocysts to provide embryonic stem cells.
- The 7 January 2007 issue of Nature Biotechnology reports the successful production of amniotic fluid-derived stem cells ("AFS"). These are present in the amniotic fluid removed during amniocentesis. With the proper culture conditions, they have been shown to be able to differentiate into a variety of cell types including
- ectoderm (neural tissue)
- mesoderm (e.g., bone, muscle) and
- endoderm (e.g., liver).
So these cells are pluripotent. Although perhaps not as versatile as embryonic stem cells, they are more versatile than adult stem cells.
Applied to humans, none of the above procedures would involve the destruction of a potential human life.
2 November 2024


