Humans (and all jawed vertebrates) have the ability to make antibodies able to
- recognize (by binding to) virtually any antigenic determinant (epitope)
- to discriminate between even similar epitopes.
Not only does this provide the basis for protection against disease organisms, but it makes antibodies attractive candidates to target other types of molecules found in the body, such as:
- receptors or other proteins present on the surface of normal cells
- molecules present uniquely on the surface of cancer cells.
Thus the remarkable specificity of antibodies makes them promising agents for human therapy. Imagine, for example, being able to
- make an antibody that will bind only to the cancer cells in a patient
- coupling a cytotoxic agent (e.g. a strong radioactive isotope) to that antibody, and then
- giving the complex to the patient so it can seek out and destroy the cancer cells (and no normal cells).
But there are problems to be solved before antibodies can be used in human therapy.
1. The response of the immune system to any antigen, even the simplest, is polyclonal. That is, the system manufactures antibodies of a great range of structures both in their binding regions as well as in their effector regions.
2. Even if one were to isolate a single antibody-secreting cell, and place it in culture, it would die out after a few generations because of the limited growth potential of all normal somatic cells.
What is needed is a way to make "monoclonal antibodies":
- antibodies of a single specificity that are
- all built alike because they are being manufactured by a single clone of plasma cells
- that can be grown indefinitely.
This problem was solved for mice in 1975 with a technique devised by Köhler and Milstein (for which they shared a Nobel Prize in 1984).
An antibody-secreting B cell, like any other cell, can become cancerous. The unchecked proliferation of such a cell is called a myeloma.
Köhler and Milstein found a way to combine
- the unlimited growth potential of myeloma cells with
- the predetermined antibody specificity of normal immune spleen cells.
They did this by literally fusing myeloma cells with antibody-secreting cells from an immunized mouse. The technique is called somatic cell hybridization. The result is a hybridoma.
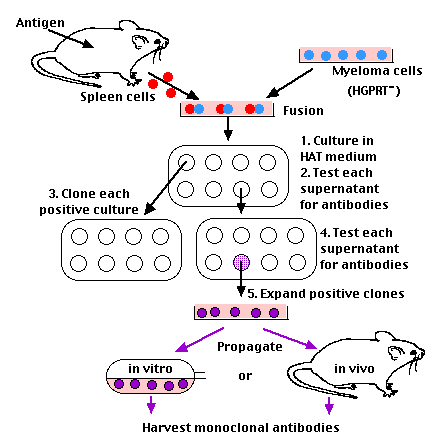 Mix
Mix
- spleen cells from a mouse that has been immunized with the desired antigen with
- myeloma cells.
Use an agent to facilitate fusion of adjacent plasma membranes.
Even so, the success rate is so low that there must be a way to select for the rare successful fusions. So,
use myeloma cells that have:
- lost the ability to synthesize hypoxanthine-guanine-
phosphoribosyltransferase (HGPRT).
This enzyme enables cells to synthesize purines using an extracellular source of hypoxanthine as a precursor. Ordinarily, the absence of HGPRT is not a problem for the cell because cells have an alternate pathway that they can use to synthesize purines.
However, when cells are exposed to aminopterin (a folic acid analog), they are unable to use this other pathway and are now fully dependent on HGPRT for survival.
- lost the ability to synthesize any antibody molecules of their own (so as not to produce a hybridoma producing two kinds of antibody molecules).
1. The first property is exploited by transferring the cell fusion mixture to a culture medium — called HAT medium because it contains:
- hypoxanthine
- aminopterin
- the pyrimidine thymidine
The logic:
- Unfused myeloma cells cannot grow because they lack HGPRT.
- Unfused normal spleen cells cannot grow indefinitely because of their limited life span. However,
- Hybridoma cells (produced by successful fusions) are able to grow indefinitely because the spleen cell partner supplies HGPRT and the myeloma partner is immortal.
2. Test the supernatants from each culture to find those producing the desired antibody.
3. Because the original cultures may have been started with more than one hybridoma cell, you must now isolate single cells from each antibody-positive culture and subculture them.
4. Again, test each supernatant for the desired antibodies. Each positive subculture — having been started from a single cell — represents a clone and its antibodies are monoclonal. That is, each culture secretes a single kind of antibody molecule directed against a single determinant on a preselected antigen.
5. Scale up the size of the cultures of the successful clones.
Hybridoma cultures can be maintained indefinitely:
- in vitro; that is, in culture vessels. The yield runs from 10-60 µg/ml.
- in vivo; i.e., growing in mice. Here the antibody concentration in the serum and other body fluids can reach 1-10 mg/ml. However, animal welfare activists in Europe and in the U.S. are trying to limit the use of mice for the production of monoclonals.
Monoclonal antibodies are widely used as diagnostic and research reagents as well as in human therapy. (It is estimated that worldwide sales of monoclonal antibodies in 2009 exceeded 36 billion dollars.)
In some in vivo applications, the antibody itself is sufficient. Once bound to its target, it triggers the normal effector mechanisms of the body.
In other cases, the monoclonal antibody is coupled to another molecule, for example
- a fluorescent molecule to aid in imaging the target
- a strongly-radioactive atom, such as Iodine-131 to aid in killing the target.
Currently (2023) some 200 monoclonal antibody preparations have either been approved by the U.S. Food and Drug Administration for use in humans or are in clinical trials. Here is a selection.
To suppress the immune system
- Muromonab-CD3 (OKT3) and two humanized anti-CD3 monoclonals. Bind to the CD3 molecule on the surface of T cells. Used to prevent acute rejection of organ, e.g., kidney, transplants. The humanized versions show promise in inhibiting the autoimmune destruction of beta cells in Type 1 diabetes mellitus.
- Infliximab (Remicade®) and adalimumab (Humira®). Bind to tumor necrosis factor-alpha (TNF-α). Show promise against some inflammatory diseases such as rheumatoid arthritis (by blunting the activity of Th1 cells). Side-effects: can convert a latent case of tuberculosis into active disease; can induce the formation of autoantibodies (by promoting the development of Th2 cells).
- Omalizumab (Xolair®). Binds to IgE thus preventing IgE from binding to mast cells. Shows promise against allergic asthma.
- Daclizumab (Zenapax®). Binds to part of the IL-2 receptor exposed at the surface of activated T cells. Used to prevent acute rejection of transplanted kidneys. Has also showed promise against T-cell lymphoma.
To kill or inhibit malignant cells
To inactivate infectious viruses
So far the use of monoclonal antibodies as a treatment for an ongoing infection has worked only for Ebola.
However, many laboratories are hastily trying to develop monoclonals that can inactivate SARS-CoV-2, the agent that is currently sweeping the world causing COVID-19.
- Vitaxin. Binds to a vascular integrin (alpha-v/beta-3) found on the blood vessels of tumors but not on the blood vessels supplying normal tissues. In Phase II clinical trials, Vitaxin has shown some promise in shrinking solid tumors without harmful side effects.
- Bevacizumab (Avastin®). Binds to vascular endothelial growth factor (VEGF) preventing it from binding to its receptor. Approved by the US FDA in February 2004 for the treatment of colorectal cancers.
Other
- Abciximab (ReoPro®). Inhibits the clumping of platelets by binding the receptors on their surface that normally are linked by fibrinogen. Helpful in preventing reclogging of the coronary arteries in patients who have undergone angioplasty.
Mouse antibodies are "seen" by the human immune system as foreign, and the human patient mounts an immune response against them, producing HAMA ("human anti-mouse antibodies"). These not only cause the therapeutic antibodies to be quickly eliminated from the host, but also form immune complexes that cause damage to the kidneys.
(Monoclonal antibodies raised in humans would lessen the problem, but few people would want to be immunized in an attempt to make them, and most of the attempts that have been made have been unsuccessful.)
However, using genetic engineering it is possible to make mouse-human hybrid antibodies to reduce the problem of HAMA.
- Chimeric antibodies. The antibody combines the antigen-binding parts (variable regions) of the mouse antibody with the effector parts (constant regions) of a human antibody. Infliximab, rituximab, and abciximab are examples.
- Humanized antibodies. The antibody combines only the amino acids responsible for making the antigen binding site (the hypervariable regions) of a mouse (or rat) antibody with the rest of a human antibody molecule thus replacing its own hypervariable regions. Daclizumab, Vitaxin, Mylotarg®, Herceptin®, and Xolair® are examples.
In both cases, the new gene is expressed in mammalian cells grown in culture (E. coli cannot add the sugars that are a necessary part of these glycoproteins).
Other ways of solving the problem of HAMA are being vigorously pursued.
Transgenic mice. One of these is to exploit transgenic technology to make transgenic mice that:
- have had human antibody gene loci inserted into their bodies (using the embryonic stem cell method).
- have had their own genes for making antibodies "knocked out".
The result is a mouse that
- can be immunized with the desired antigen
- produces human, not mouse, antibodies against the antigen
- can yield cells to be fused with myeloma cells to manufacture all-human monoclonal antibodies.
Phage display is another technique for making all-human monoclonal antibodies. Link to discussion.
Antibodies can bind to molecules expressed at the surface of target cells (as well as to soluble molecules) but are not effective against the peptide fragments that antigen-presenting cells contain tucked within their histocompatibility molecules. T-cell receptors are the ligands needed for that job. [Discussion]
So monoclonal antibodies are not effective against intracellular antigens, e.g. virus-encoded proteins and tumor-specific antigens. But now progress is being made toward the development of monoclonal T-cell receptors (αβ TCRs).
Two ways in which these molecules could be helpful:
- Transforming normal T cells — whatever their natural specificity — so they also express a new TCR of desired specificity (and high affinity). These could then be introduced into a cancer patient to target the tumor-specific antigens [An example] or into an AIDS patient to target HIV-infected cells.
- Preparing a fusion protein of
to destroy cells expressing the target MHC-peptide complex.
Look forward to clinical trials.
4 January 2023
 Mix
Mix