| Index to this page |
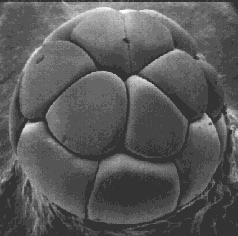
Mitosis and cytokinesis of the zygote, an unusually large cell, produces an increasing number of smaller cells, each with an exact copy of the genome present in the zygote. However, the genes of the zygote are not expressed at first. The early activities of cleavage are controlled by the mother's genome; that is, by mRNAs and proteins she deposited in the unfertilized egg. In humans, the switch-over (called zygotic-genome activation, ZGA) occurs after 4–8 cells have been produced; in mice at the two cell stage; in frogs not until thousands of cells have been produced. Cleavage ends with the formation of a blastula.
| More on cleavage (with illustrations) |
There is little visible differentiation of the cells in the various layers, but probes for cell-specific proteins reveal that different groups of cells have already started on specific paths of future development.
Gastrulation forms three major "germ layers": ectoderm, mesoderm, and endoderm. By gastrulation, the genes of the zygote genome are being expressed.
| View these stages as they occur in amphibians such as Xenopus laevis, the South African clawed frog. |
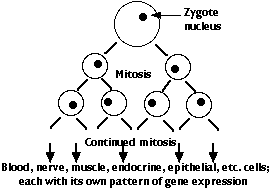 The genome of the zygote contains all the genes needed to make the hundreds of different types of cells that will make up the complete animal. There are two major categories of these genes:
The genome of the zygote contains all the genes needed to make the hundreds of different types of cells that will make up the complete animal. There are two major categories of these genes:
However, every cell descended from the zygote has been produced by mitosis and thus contains the complete genome of the organism (with a very few exceptions).
Two pieces of evidence:
| How Dolly was made |

Spemann's egg-tying experiments provided a clue.
When he repeated his experiments with the fertilized egg constricted so that all of the gray crescent lay in one half, the final results were quite different from what we saw above. The half lacking the gray crescent but containing the nucleus began cleavage right away, yet it never developed beyond an unorganized mass of intestinal, liver, and other abdominal cells (left). The other half, even though it did not get a nucleus until the fourth mitotic division on the cleaving side, went on to form an perfectly normal embryo. This again was proof that the nuclei, here at the 16-cell stage, had not lost any genes. But why, then, did a normal embryo fail to develop on the side with the original zygote nucleus?
The distribution of the cytoplasmic contents — mitochondria, RNA, ribosomes, yolk, etc. — in the amphibian egg is not uniform. Shortly after fertilization, some of the cytoplasmic constituents migrate and form the gray crescent. In Spemann's first experiment, each half of the egg contained all the normal egg constituents because the longitudinal constriction was perpendicular to the gray crescent. However, in the second experiment, the hemisphere lacking the gray crescent must have lacked some essential cytoplasmic materials. So his work provided an early clue that the potentialities a nucleus can achieve are regulated by the cytoplasmic environment in which it finds itself. Now let us bring the story up-to-date.

The photo on the left (courtesy of Douglas Melton, in whose lab the work was done) shows the tadpole that developed from a frog embryo that had been injected with mRNA for activin. At the 32-cell stage of cleavage, a single cell — that would normally have gone on to form part of the belly of the animal — was injected with activin mRNA. A two-headed tadpole resulted (arrow). (A normal tadpole is shown on the right for comparison.)
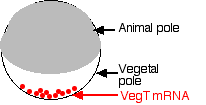
The mother is also responsible for making ectoderm. During egg formation, she deposits mRNAs encoding ectodermin at the animal pole of the egg. Ectodermin interferes with SMAD signaling and thus prevents TGF-β proteins from forming mesoderm at the animal pole.
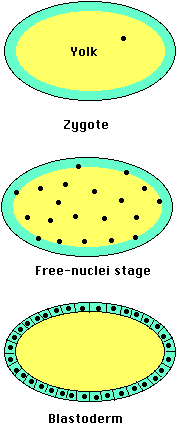
In Drosophila and other insects, cleavage involves repeated mitosis but without cytokinesis (forming a syncytium). So the daughter nuclei remain suspended within the single egg compartment. After several thousand nuclei have been formed, they migrate to the margins of the egg. Only then do plasma membranes form around each nucleus forming true cells. But, as for Xenopus, the genes that will be expressed by those cells are regulated by the cytoplasmic constituents they found themselves surrounded by. And that, again like Xenopus, is determined by where those molecules end up in the egg.
For example, Drosophila eggs have a gradient of mRNA transcribed from a gene designated bicoid (bcd). The transcripts are deposited in the egg by "nurse" cells surrounding it. Once within the egg, they are transported (along microtubules) toward the anterior. The result is a concentration gradient of bicoid mRNA extending from a high level at the anterior of the egg to a low level at the posterior.
After fertilization, the mRNAs are translated into bicoid protein. High levels of the protein lead to the formation of the head of the larva.
Conversely, the posterior of the egg has a high concentration of mRNA encoding the nanos protein, which is needed to form the structures of the tail of the larva.
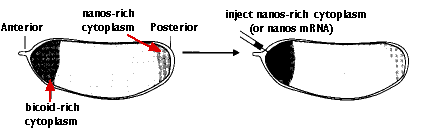
Inject the anterior of the fertilized egg with nanos mRNA. The result: another double-posterior larva.
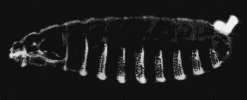
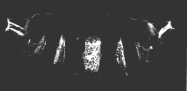
A normal larva is shown on the right. The bright object at the right end of the normal larva and at both ends of the double posterior larva is the tip of the tail. These micrographs are courtesy of Elizabeth Gavis and Ruth Lehmann, in whose lab the third demonstration was performed.
The mud snail, Ilyanassa obsoleta, is a small gastropod that lives in mud flats along the Atlantic coast.
Like other protostomes, cleavage of the zygote produces daughter cells that are already committed to their fate. In other words, even as early as the two-cell stage, the cells are no longer totipotent. Unlike humans and other deuterostomes, then, identical twins cannot form.
In the 12 December 2002 issue of Nature, J. David Lambert and Lisa Nagy reported another mechanism by which two daughter cells become committed to different fates even though they have inherited the same genome.
They traced the distribution in the cells of early embryos of the messenger RNAs (mRNAs) encoding 3 proteins that are known to be important in the development of other animals such as Xenopus and Drosophila.One of the first events when an animal cell prepares to divide by mitosis is the duplication of its centrosome and separation of the duplicates. [Link to discussion.]
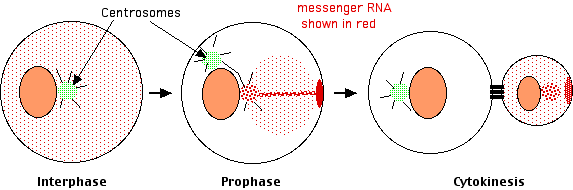 Lambert and Nagy found that
Lambert and Nagy found that
Centrosome sorting (of proteins in this case) also plays a role in determining whether embryonic cells of Caenorhabditis elegans remain in the germline or become the somatic cells of the worm. [Link to discussion.]
| see Organizing the Embryo: The Central Nervous System |
| see Organizing the Embryo: Segmentation |
| see Embryonic Development: Putting on the finishing touches |
| Welcome&Next Search |